LabSoft LIMS software not only handles the "nuts and bolts" functionality to satisfy your ISO, GLP requirements,
but adds powerful reporting, plotting and viewing tools not found in any other LIMS system.
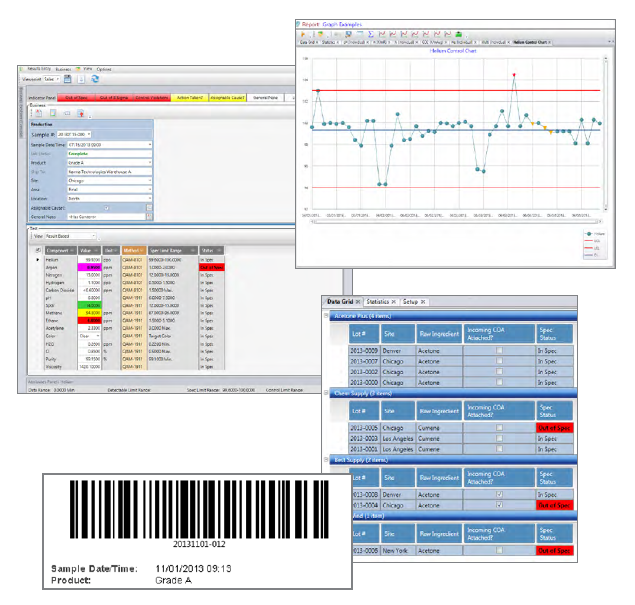 Manage Samples
Manage Samples
Uses a flexible configurable "LogBook" concept to provide a facility to enter, update and view
samples, test results and supporting notes.
Tools
Vast range of
reporting tools used to "read" data from the database.
Notices
An E-mail type message system that
alerts users of important LIMS events.
Certificates of Analysis
Create COAs and manage COA requirements
Specifications
An integrated Agreement module manages specs from many different viewpoints such as manufacturing
product specs and customer specs. Complete Agreement history is maintained to ensure that past test
results will always be compared to their specs as they were when the results were entered. Extensive
supporting information is available such as who submitted the Agreement, who approved the Agreement,
notes, and others.
Configurability/Security
Adapt LabSoft LIMS to your needs by modeling it to match your company's business rules, workflow,
nomenclature, and manufacturing process.
Representative
Optional feature that allows you to select the most representative data of a sample. One test value can be 'marked' as
the representative value or multiple test values can be used to calculate an average. All data, whether
marked representative of not, is stored in the LabSoft LIMS database and can be accessed by various tools.
Methods
Provides organized storage and retrieval of test method procedures. Applicable Methods can be viewed by the
lab technicians as needed. Methods can optionally be embedded in the LabSoft LIMS database or linked to
existing files.
Notes
Augment samples/test results by entering General Notes, Action Taken events, and Assignable Cause events.
General Notes are used for many purposes by lab technicians, lab managers, and control room operators to document events
and conditions. Action Taken Notes are used to document specific corrective actions (i.e. adjustments, reworks) taken in
the process. Assignable Cause Notes provide a structured coded methodology and unlimited free text to identify Assignable
Cause versus Common Cause process variation. Assignable Cause data can be excluded when graphing, reporting, or
calculating statistics.
Statistics
Integrated throughout LabSoft LIMS. Statistical information is available to aid in SPC (Statistical Process Control).
Bar Codes
Configurable Bar Code label printing.
E-mail
Send E-mails directly from LabSoft LIMS with information such as Lab Results, Reports, Statistics,
Notes, Control Plans. COAs, etc.
ISO 9000/GLP/Traceability
All significant events are recorded in an Audit Trail. All revisions to LogBooks, Specifications, COA
templates, and Result Values are maintained for traceability and to assure ISO 9000 and GLP compliance.
Each individual Result Value is stored with the user who entered it.
Accessibility
Because LabSoft LIMS is based on an open non-proprietary database architecture, the data is available
to 3
rd party applications via ODBC or ASCII comma delimited files.
Microsoft compatibility
Multi-system
Data can be extracted from multiple LabSoft LIMS systems across a LAN/WAN. This powerful feature allows you to
mix/match/correlate results from "sister" or "feed stock" sites.
Multi-user
Context sensitive help
Add-Ins Avaliable
Dynamics AX
External Data for PI
External Data for IP.21
DataPort
Azure Storage